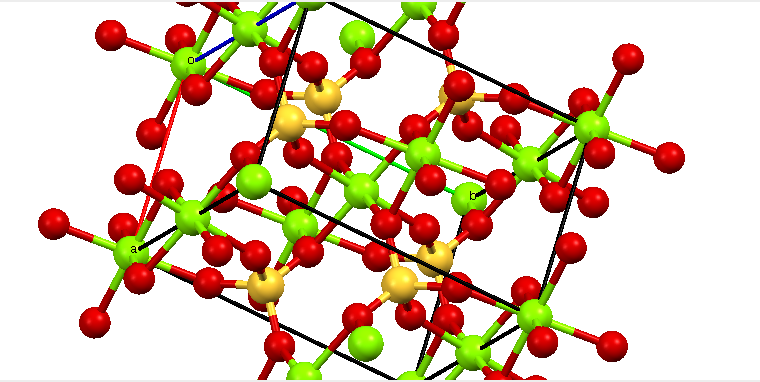
MgSO₄

Photo of MgSO₄
Epsom Salt!
Magnesium Sulfate, MgSO₄, Is an inorganic magnesium salt.
It Is an Ionic Solid, and is both colorless and odorless.
Its Uses include:
- Increasing magnesium and sulphur within the soil
- Used as a coagulant to make tofu
- Used in Iv to control seizures
Magnesium Sulfate also have countless uses in pharmacy, being parts of many different pharmaceutical drugs.
.png)


.png)